Question 8
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii)CH2FCO2H or CH2ClCO2H
(iii) CH2FCH2CH2CO2H or CH3CHFCH2CO2H
(iv)
Question 1
What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin (ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiacetal
(vi) Oxime
(vii) Ketal
(vii) Imine
(ix) 2,4-DNP-derivative
(x) Schiff's base
Question 2
Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO-p
Question 7
Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal (ii) 2-Methylpentanal
(iii) Benzaldehyde (iv) Benzophenone
(v) Cyclohexanone (vi) 1-Phenylpropanone
(vii) Phenylacetaldehyde (viii) Butan-1-ol
(ix) 2, 2-Dimethylbutanal
Question 8
How will you convert ethanal into the following compounds?
(i) Butane-1, 3-diol (ii) But-2-enal (iii) But-2-enoic acid
Question 12
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Question 14
How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom
(i) Methyl benzoate (ii) m-Nitrobenzoic acid
(iii) p-Nitrobenzoic acid (iv) Phenylacetic acid
(v) p-Nitrobenzaldehyde.
Question 16
Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
Question 17
Complete each synthesis by giving missing starting material, reagent or products
(i)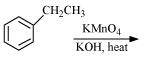
(ii)
(iii)
(iv)
(v)
(vi)
Question 18
Give plausible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6 trimethylcyclohexanone does not.
(ii) There are two -NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.